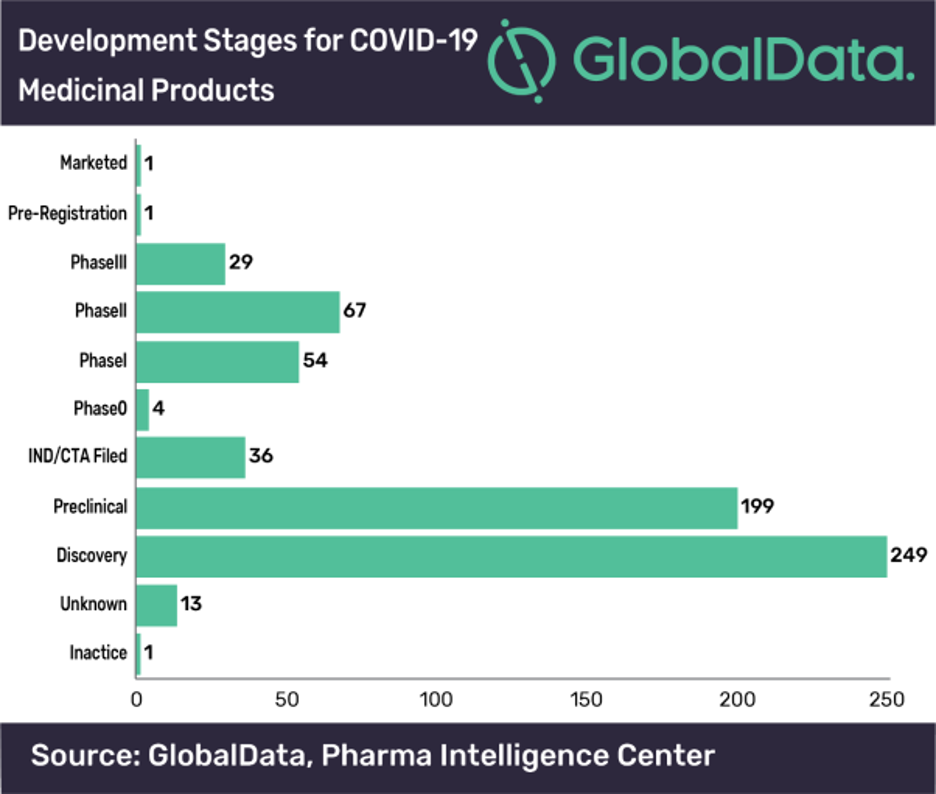
A large number of COVID-19 treatment options (620 novel and repurposed drugs as of mid-May) have joined the drug development pipeline, with over 72% of these drugs in preclinical or discovery stages of development. These drugs are in the initial stages of feasibility and drug safety investigation to show that they have an effect on COVID-19 and are tolerable to administer, according to GlobalData, a leading data and analytics company.
Gilead’s remdesivir reached the market in Japan in mid-May and is in pre-registration in the EU. The drug is the new standard of care, and has earned an Emergency Use Authorization (EUA) in the US. It is approved in Japan for the treatment in hospitalized patients with COVID-19.
Johanna Swanson, Product Manager at GlobalData comments: “As of 19th May, the drugs in Phase III for COVID-19 included some that were already marketed for other diseases such as Alexion Pharmaceuticals’ eculizumab, with US sales of $3.56bn; Novartis/Incyte’s JAK inhibitor ruxolitinib phosphate, with global sales of $2.36bn; Roche’s tocilizumab, with global sales of $2.21bn; Novartis’ canakinumab with global sales of $554m; Eli Lilly’s baricitinib, with US sales of $203m; Swedish Orphan Biovitrum’s anakinra, with global sales of $151m; Regeneron Pharmaceuticals’ sarilumab, with global sales of $109m; and Mesoblast Ltd’s remestemcel-L, with global sales of $18m.
“These drugs already had a manufacturing and supply chain, so they are more likely to be able to provide a treatment option if they pass their Phase III clinical trials for efficacy and safety.
“The molecule types of the drugs being worked on show a high number of biologics (412) and vaccines (176) in the pipeline for development. A large number of competing vaccines is good. This increases the odds of achieving a successful vaccine, as the virus’ components are poorly understood, making it difficult to determine which parts might be more immunogenic and thus likely to lead to a successful vaccine. Additionally, some of these potential COVID-19 vaccines are being produced with methods that are untested at this scale of manufacture.”